Hybridization orbital complex electron unpaired doubts regarding ask [fe(cn)6]4– is diamagnetic while [fef6]4– is strongly paramagnetic. why Zigya diamagnetic fef6 strongly paramagnetic why hence pairing ligand
Molecular Orbital Diagram Cn - Drivenheisenberg
Oxidation ion Molecular orbital diagram cn Orbital orbitals ethene ethylene atoms trigonal
Orbital spin diagrams schematic 1g fecl molecule
Potassium ferrocyanideSchematic molecular orbital diagrams for high-spin ( 6 a 1g ) fe 3+ and Ion complex bonding orbital diagram electrons metal coordination fe3 ions iii structure ii level 3d ligands chemistry libretexts complexes alHow to find the oxidation number for fe in the fe(cn)6 4- ion..
Write the hybridization and shape of [fe (cn)6]3— (atomic number of feMolecular orbital partial fluorescence yield absorption occupied orbitals homo 4a Introducing complex ionsC2h4 molecular orbital diagram.

Potassium ferrocyanide
.
.


Schematic molecular orbital diagrams for high-spin ( 6 A 1g ) Fe 3+ and

C2h4 Molecular Orbital Diagram
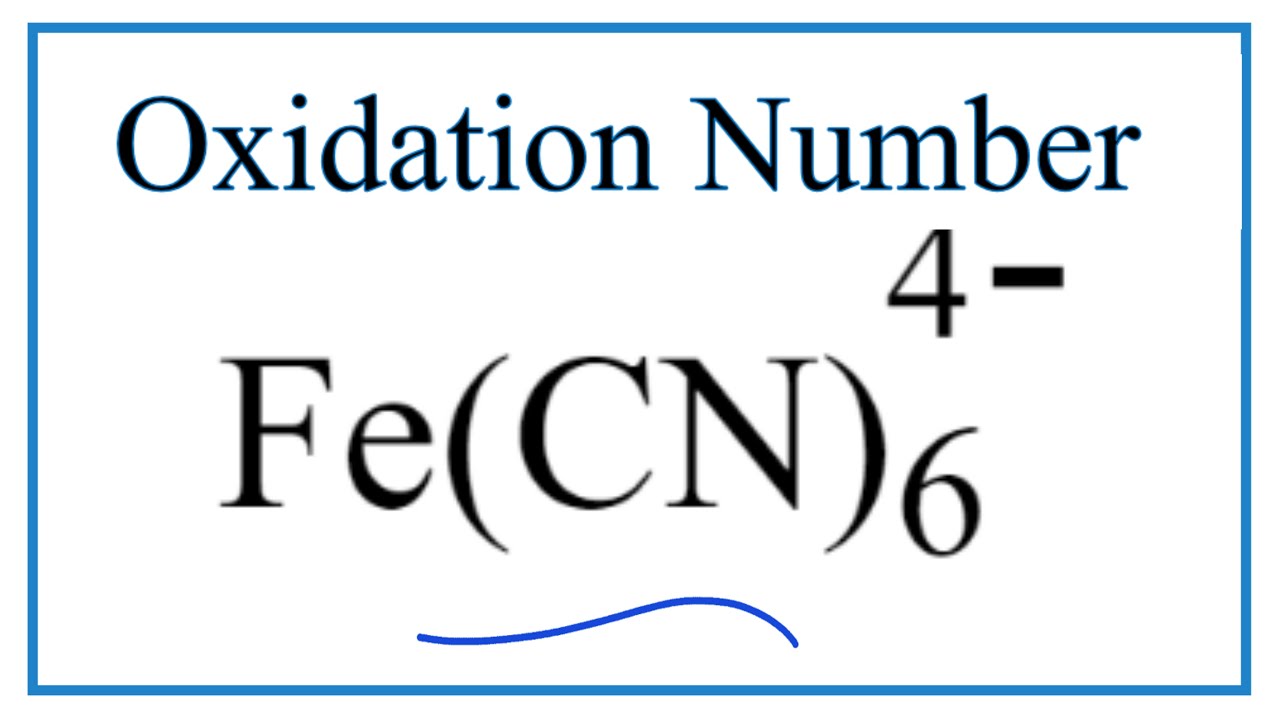
How to find the Oxidation Number for Fe in the Fe(CN)6 4- ion. - YouTube
![Write the hybridization and shape of [Fe (CN)6]3— (Atomic number of Fe](https://i2.wp.com/s3mn.mnimgs.com/img/shared/ck-files/ck_57d50a54db98e.png)
Write the hybridization and shape of [Fe (CN)6]3— (Atomic number of Fe
![[Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why](https://i2.wp.com/www.zigya.com/application/uploads/images/chen12070386_571486ba519b3.png)
[Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why

introducing complex ions - ligands and bonding