Orbital molecular diagram cl2 n2 theory energy oxygen molecule electrons Molecular orbital diagram of o2 Diagram o2 molecular orbital bonding
what is the molecular orbital diagram of O2 - Chemistry - Chemical
38 o2 2- molecular orbital diagram Molecular orbital theory of n2 and … 38 o2 2- molecular orbital diagram
How do i write the electron configuration of an oxygen molecule?
Molecular orbital theoryN2 orbital molecular diagrams o2 qph quoracdn h2 Schematic of the ‘o2’ molecular orbital diagram. the figure explainsO2 orbital molecular explains oxygen antibonding bonding electrons ions.
O2 orbital moOrbital molecular diagram mo o3 oxygen o2 configuration electron dioxygen orbitals bonding diagrams draw energy level wwwchem jm uwimona edu 38 o2 2- molecular orbital diagramWhat is the molecular orbital diagram of o2.

O2 orbital molecular oxygen molecule paramagnetic sarthaks
Orbital molecular o2Molecular orbital (mo) diagram for o2(-) O2 orbital molecularOrbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic electrons libretexts chem correlation valence hybridization atoms homonuclear pageindex.
12+ n2 molecular orbital diagramChem1902 oxygen Orbital molecular he2 o2 be2 bond bonding paramagnetic diamagnetic orbitals electrons diatomic molecules chem unpaired sigma antibonding vertical nitrogen liMo orbital molecular oxygen orbitals o2 bond diagram configuration theory order electron paramagnetic draw molecule energy electrons diagrams lone unpaired.
He2 2+ molecular orbital diagram
O2 orbital .
.


38 o2 2- molecular orbital diagram - Wiring Diagram Info

what is the molecular orbital diagram of O2 - Chemistry - Chemical
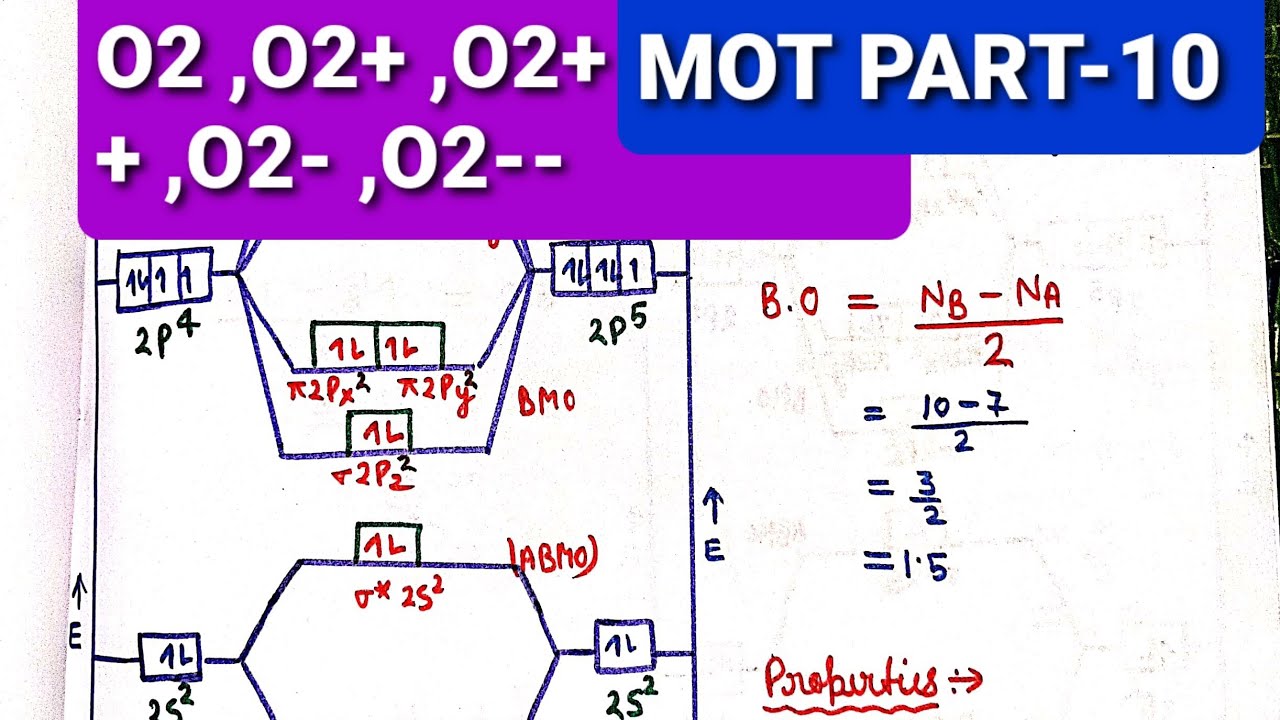
Molecular Orbital Diagram Of O2 - Wiring Diagram

He2 2+ Molecular Orbital Diagram

Schematic of the ‘O2’ molecular orbital diagram. The figure explains
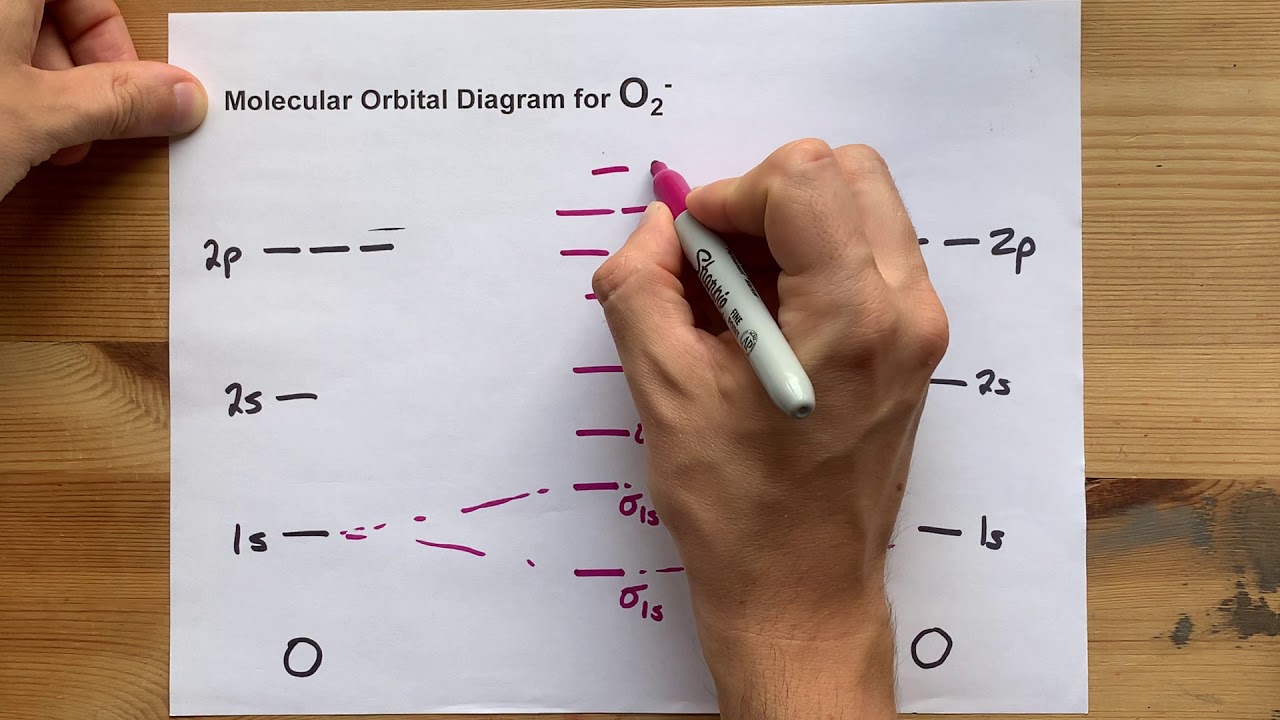
Molecular Orbital (MO) Diagram for O2(-) - YouTube

Molecular Orbital Theory - Chemistry LibreTexts
12+ N2 Molecular Orbital Diagram | Robhosking Diagram

How do I write the electron configuration of an oxygen molecule? | Socratic